Overjet has received FDA 510(k) clearance for its CBCT Assist tool, expanding its AI platform to support diagnostic interpretation of 3D dental imaging.
Overjet has received 510(k) clearance from the U.S. Food and Drug Administration for its CBCT Assist product, expanding the company’s AI platform to include diagnostic support for cone beam computed tomography (CBCT) imaging.
AI Support for CBCT Imaging
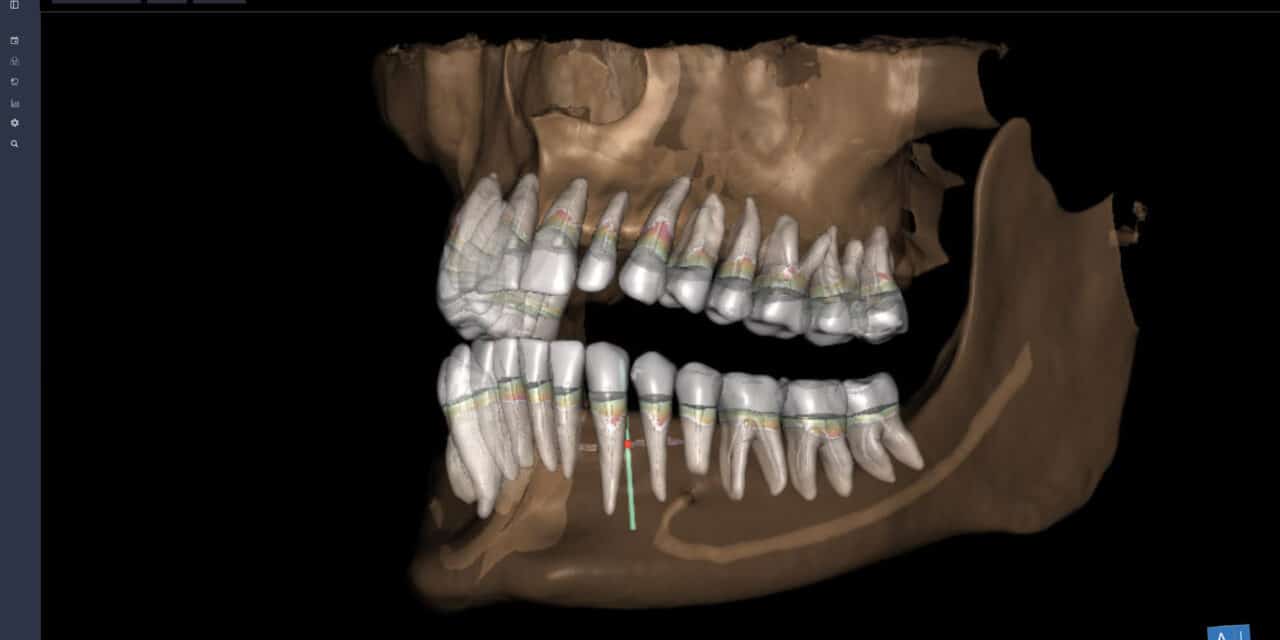
The new product assists dental professionals in the review and interpretation of CBCT images. It enables anatomical assessment and supports diagnostic and treatment planning by automatically identifying, labeling, segmenting, and quantifying key anatomical and restorative structures in CBCT scans. Clinically relevant measurements provided include periodontal bone levels, airway minimal cross-sectional area, distances between tooth and anatomical structures, and tooth impaction ratio.
Image Enhancement and Measurement Capabilities
CBCT Assist includes image enhancement tools such as brightness, contrast, and Virtual Panoramic reconstruction. It also features measurement tools for distance, area, angle, and signal intensity.
Clinical Applications
The product supports advanced visualization with Multi-Planar Reconstruction views and 3D Volume Rendering. It facilitates surgical planning, including third molar surgery, by generating linear measurements to nearby critical structures. It also aids in implant site evaluation by providing measurements for assessing bone availability and anatomical features. For airway assessment, it automatically reports the minimum cross-sectional area.
Company and Clinical Leadership Comments
“This FDA clearance for CBCT Assist, Overjet’s 10th FDA clearance to date, is a critical milestone that reinforces our leadership in bringing clinically validated AI to the forefront of oral healthcare,” said Dr Wardah Inam, CEO of Overjet. “As the industry standard for dental AI, we are committed to giving providers the best tools available to drive evidence-based treatment and ensure the highest quality of care for their patients.”
Dr Parag Kachalia, head of clinical practice at Overjet, added, “The FDA clearance for CBCT Assist is more than just a regulatory achievement; it is validation of our foundational scientific rigor. This innovation directly supports our mission to provide unparalleled diagnostic clarity and elevate the quality of care across the entire dental ecosystem.”
Photo: Overjet