The FDA granted 510(k) approval to Laon Medi’s AI-based Align Studio clear aligner treatment and software.
Laon Medi, an artificial intelligence (AI) medical solution company, announced that its dental clear aligner software, Align Studio, received 510(k) approval from the United States Food and Drug Administration (FDA). The platform reduces simple repetitive work and time-consuming processes by using deep-learning technology.
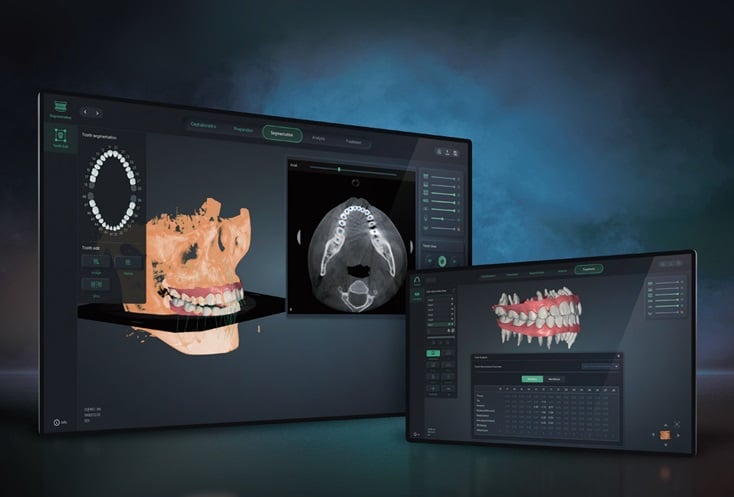
Align Studio enables real-time diagnosis and device fabrication for clear aligners based on dental scan data from CTBC and X-ray images. It separates teeth from CBCT images automatically and simulates the movement and changes of inter-tooth roots dynamics to avoid collision during the orthodontic treatment.
Align Studio is designed to reduce the time and cost involved in patient visits and diagnoses through automated analysis of images to help dental professionals create treatment plans, according to the company.
With this FDA approval, Laon Medi plans to add further AI tools to the platform. The company also plans to expand platform service to allow dental professionals working from various locations to use the software.
Photo courtesy of Laon Medi