Summary: DentalMonitoring received De Novo approval from the FDA for its AI-enabled software to optimize orthodontic care. This is the first AI-based dental software to receive such approval. The solution aids in remote monitoring and communication, enhancing treatment efficiency and patient convenience.
Key Takeaways:
- FDA De Novo Approval: DentalMonitoring is the first AI-enabled dental software to receive De Novo approval from the FDA.
- Remote Monitoring: The software assists in remotely monitoring dental and orthodontic treatments, improving patient accountability and communication.
DentalMonitoring has received De Novo approval from the U.S. Food and Drug Administration (FDA) for the DentalMonitoring solution to be used by orthodontists in optimizing clinical care.
AI-Driven Dental Software
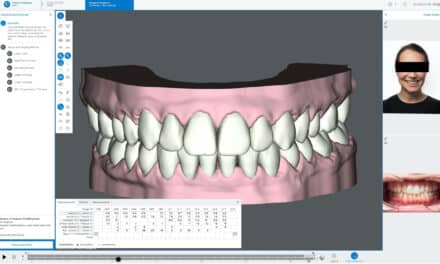
DentalMonitoring is a medical device software using image processing algorithms to analyze pictures of the oral cavity. Scans are taken using the DM App, a smartphone, and the manufacturer’s proprietary hardware products. DentalMonitoring is indicated for use for patients over the age of 6 and reports results solely on permanent teeth.
FDA Approval
Since 2012, only four De Novos have been granted by the FDA Dental panel, and none were software-based devices. DentalMonitoring is the first artificial intelligence/machine learning-enabled software as a medical device in dentistry, according to the company, establishing a new classification within FDA dental regulation numbers and product codes.
AI Imaging Analysis
The product is designed to assist healthcare professionals in remotely monitoring dental treatments, orthodontic treatments, oral health, and treatment progress. The results of DentalMonitoring are intended to be used as an aid in diagnosis and not on a stand-alone basis for clinical decision-making. With DentalMonitoring, clinicians can receive insight into treatment progress while patients enjoy increased convenience. Automated notifications and in-app communication with practice staff increase patient accountability and improve communication between patients, healthcare providers, and caregivers, according to the company.
Introducing SmartSTL Feature
With this approval, DentalMonitoring will also introduce the innovative SmartSTL feature to North American orthodontist customers. SmartSTL allows doctors to request updated STL files via the DentalMonitoring dashboard without bringing the patient back into the office.
“This is a recognition of our 10 years of research and development in Deep Learning Artificial Intelligence,” said Philippe Salah, chief executive officer and co-founder of DentalMonitoring. “FDA approval of this improved version of DentalMonitoring software is a key milestone for DentalMonitoring and marks the beginning of a new era in orthodontics.”