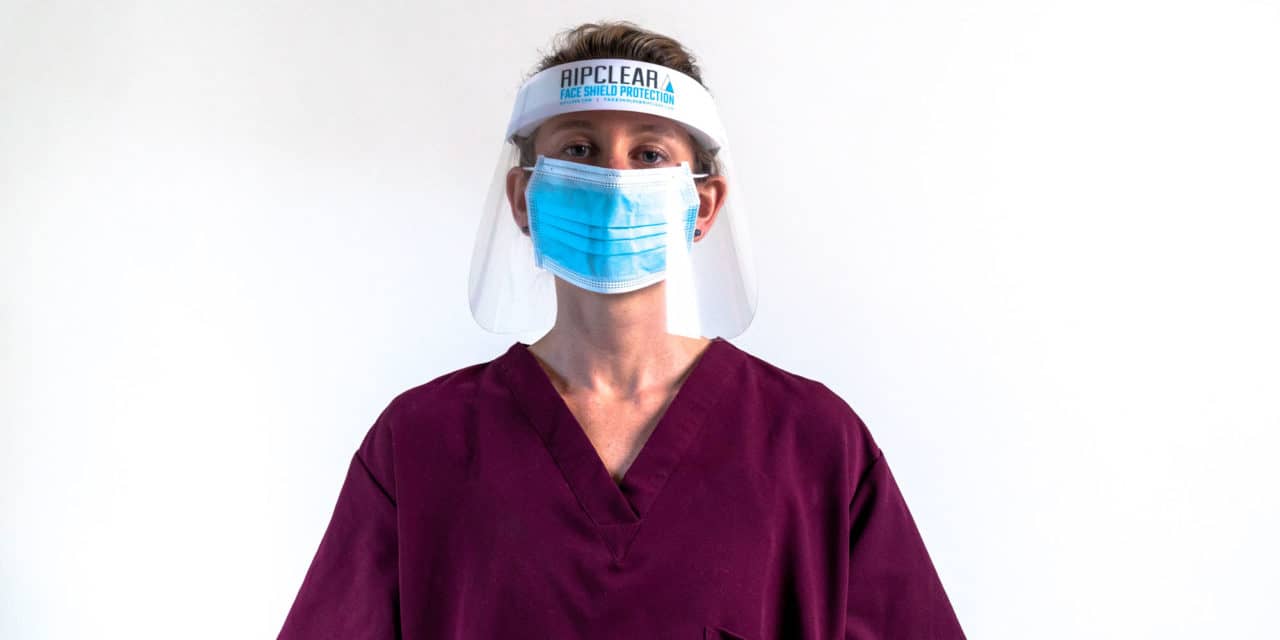
The foam padded face shield from Ripclear, which features 91% optical transparency and anti-fogging properties, has received medical device certification from the FDA.
Ripclear, makers of protective film for outdoor sports eyewear, announced the launch of its V2 Shield. Designed to protect personnel battling the COVID-19 outbreak, including EMS workers, nurses, police, and other front-line staff, the V2 Shield reportedly features 91% optical transparency, so that personnel can safely do their work while staying protected via a certified medical face shield.
In addition to its optical transparency, the foam padded face shield has an elastic band to secure it. Featuring a dual-sided anti-fog treatment, the V2 Shield is designed to protect against spray and droplet infection.
“We are taking orders of quantities anywhere from 100 units to over 1 million units directly on our site and hope to keep as many people protected from the spread of COVID-19 as humanly possible,” said Ryan Doherty, the co-founder and president of Ripclear.
Each shield measures 32 cm x 22 cm and is 0.3 mm in thickness. The face shields are stackable and travel easily.
The V2 Shield has received medical device certification from the FDA, a certificate of compliance from the CE, and a quality management system certificate from the ISO 9001.
According to the company, pricing for the V2 Shield runs from $3.50 per shield to $2.55 a shield, depending on the quantity ordered.
Ripclear is the maker of protective films for snow googles, sunglasses, action cameras, sports visors, motorcycle goggles, and smartphone and tablet screen protectors.
When the COVID-19 outbreak hit, the company initially released its Ripclear optics shield. After that proved to be a success, and realizing there was a niche to fill, the company pivoted to designing the V2 Shield to serve as a certified shield for frontline personnel exposed to COVID-19.