by Jason B. Cope, DDS, PhD
Important considerations for higher miniscrew success rates
 |
In the 9 years that I have lectured and written about temporary anchorage devices (TADs), particularly in the past 3 years, I have seen a growing interest among orthodontists in improving their success rate with miniscrew implants (MSIs). Unfortunately, too many speakers liken placing MSIs to sticking a screw into wood, which is tantamount to failure. Others, realizing my formal PhD training is in bone biology, have inquired why my success rate is higher than average. Here are some easy ways to increase the success rate of MSIs, all of which are based on basic bone biology.
1) How do you define success with regard to miniscrew implants?
Several authors have defined success and failure concerning the use of TADs in general and MSIs in particular. Unfortunately, there has been no consistency with the definitions. Moreover, the specific protocol for placement and loading is also inconsistent. This means that the results from one study cannot be directly compared to another study. Yet this comparison is all too frequently made. For example, Miyawaki defined success as a TAD that could be used for 12 months.1 What if it needs to be used for 18 months, but comes out after 13 months? According to Miyawaki, it can be considered a success even though it failed in its purpose. On the other hand, Cheng defined one criterion for success as absence of inflammation.2 What if the MSI has inflammation but it was used for 18 months as it was intended? According to Cheng, it is considered a failure even though it was successfully used in its purpose. It follows that we need to clearly define success and failure, which I have done below so that it gives us a benchmark for future studies.
 |
| Jason B. Cope, DDS, PhD |
I define a failure as any MSI displaying significant clinical mobility, rendering it incapable of completing its intended purpose in tooth movement. Based on this definition, if an MSI is needed for 3 months and can be used for 3 months, it is a success. If an MSI is needed for 18 months but can only be used for 17 months, it is a failure. For the questions that follow, I will refer to my first 103 consecutive MSIs that were placed, used, and removed. Of these, one oral surgeon placed the first nine MSIs, another oral surgeon placed the second two MSIs, and I placed the remaining 92 MSIs. Collectively, we had an 89.3% success rate. Independently, we had success rates of 77.8%, 50%, and 91.3%, respectively.
2) What is primary stability, and how does it affect success?
Implant mobility can be divided into two periods: immediate and delayed. Immediate mobility upon placement is also referred to as inadequate primary stability. Delayed mobility, which occurs days to months after placement, is a separate entity that is referred to as inadequate secondary stability. This type of mobility is usually caused by implant overloading.
Research has clearly demonstrated that primary stability is critical for MSIs. Primary stability refers to the lack of movement of an MSI upon initial placement. This is determined by gently, but firmly, pushing on the MSI from one direction with a ligature director and feeling no “give” by the MSI. A lack of primary stability almost routinely leads to overt MSI mobility, with subsequent failure. Recent evidence suggests that the majority of primary MSI stability comes from cortical bone, with lesser stability coming from medullary bone.3 This complication basically results from inadequate cortical bone around the MSI. Causes of inadequate primary stability can be divided into three major categories: 1) inadequate bone stock (see question 3), 2) pilot hole overenlargement (see question 4), and 3) pilot drill overheating (see question 4).
Upon placement, an MSI should have at least 0.5 mm to 0.75 mm of available bone stock around its circumference. If not, the MSI has an increased risk of failure. Recently, several authors have provided data outlining predictable intraalveolar and extraalveolar sites that provide adequate bone stock for MSI placement (see question 3).4-6 If an MSI is placed in a region of questionable cortical bone thickness or density, such as adjacent to a pneumatized sinus or a recent extraction site, and the MSI feels even subtly mobile, then a new location should be considered. Because most MSIs are intended to be placed and loaded at the same visit, the MSI should have adequate cortical bone purchase and exhibit no mobility. If mobility is present at the time of placement, it will never get better; the MSI should be relocated immediately.
In my first 103 MSIs, MSIs that had good primary stability demonstrated a 91% success rate. Those MSIs without primary stability demonstrated a 0% success rate.
3) Are there locations in the mouth that contribute to a higher success rate?
Although it may not be readily apparent, MSIs can be placed just about anywhere in the oral cavity where there is adequate bone. The key to success is in placing the MSI in a location with adequate bone, both in terms of cortical thickness and circumference around the MSI. In the maxilla, several intraalveolar and extraalveolar locations are available for MSI placement. Recent evidence suggests that the majority of primary stability comes from the cortex,3 specifically where the threads engage the cortical bone. Although cortical bone thickness varies from patient to patient, certain sites provide rather consistent cortical bone thicknesses.7
One of the most reliable intraalveolar maxillary buccal cortical sites is found mesial to the first molar. This is followed by the interradicular space between the canine and lateral incisor.4 The palate also provides multiple sites for MSI placement. The posterior palatal alveolar interradicular sites mirror their corresponding buccal counterparts, yet have slightly more available bone because of their solitary palatal roots. The midpalatal area provides considerably more cortical bone than the alveolus. In the midpalate, midsagittal placement is suitable in nongrowing adults; however, growing children should have MSIs placed parasagittally instead. A great maxillary extraalveolar location for MSI placement is the infrazygomatic crest (IFZC), which is the sweeping arc of bone palpable high in the maxillary vestibule adjacent to the first and second molars.
In the mandible, several intraalveolar and extraalveolar locations are available. Although the facial and lingual surfaces of the alveolar bone are candidates, the lingual aspect is difficult to access and is in a location that predisposes it to premature failure because of the activity of the tongue musculature. One of the most reliable mandibular buccal cortical sites is found mesial to the first molar. This site is followed by the interradicular space between the canine and lateral incisor.4
A great extraalveolar location for MSI placement is the posterior mandible. In this region are three distinct sites. MSIs can be placed obliquely in the external oblique ridge lateral to the first and second molars for intrusion or retraction mechanics. Similarly, the ascending ramus or the retromolar region can be used for retraction or molar uprighting.
Interestingly, in the upper and lower arches, I have a 100% success rate for MSIs placed anterior to the first premolar and an 86.8% success rate posterior to the first premolar. In addition, for MSIs placed in the palate, my success rate is 95%.
4) Are there variations in MSI design that affect success rates?
A distinction should be made regarding screw-shaped implant nomenclature. Self-tapping simply refers to the ability of an MSI to advance when turned while creating its own thread in bone. There are two types of self-tapping MSIs: thread-forming and thread-cutting. Both MSI types have sharp threads for forming the internal bony threads during MSI advancement. In addition, a thread-cutting screw has a notch cut parallel to the long axis and located at the apex of the MSI, so that as the MSI is advanced into the bone, the notched surface of the MSI actually cuts or taps and removes bone out of the way of the advancing threads.8 The notch also aids in removal of swarf, or bone debris, created during MSI placement. Importantly, the notch feature may tend to weaken smaller screw-shaped implants, increasing the risk of fracturing the tip on placement. Moreover, when the MSI is left in place for long periods, bone may have grown into the notch, thereby making MSI removal difficult.9,10
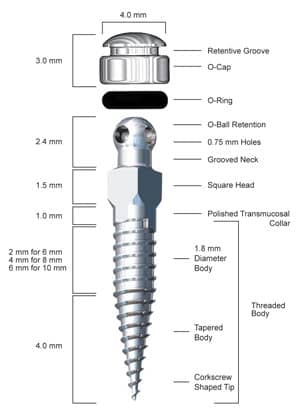 |
| Figure 1: The IMTEC Ortho Implant. |
A self-drilling or drill-free MSI is one in which its apex is sharp and tapered to allow for placement without a predrilled pilot hole. For example, the Ortho Implant (from IMTEC, A 3M Company, Ardmore, Okla) is drill-free (Figure 1) and self-tapping (thread-forming). Recent evidence suggests that drill-free MSIs have better bone-to-implant adaptation than non–drill-free MSIs.11
Finally, a non–drill-free MSI requires the placement of a pilot hole before MSI insertion either because the apex is not sharp enough to perforate the outer bony layer or because the MSI is too small in diameter to allow placement into bone without the possibility of fracture.
Importantly, there are several reasons to avoid an MSI that requires a pilot hole. An overdrilled pilot hole may lead to inadequate primary stability.12,13 This problem is more probable in thin cortical and soft cancellous bone.14,15 The main reason for hole overenlargement is inability to hold the handpiece stable and perpendicular to the bone surface during drilling. Any angular movement other than straight up and down during drilling will enlarge the pilot hole and minimize the tight fit of the MSI in the pilot hole.
Excessive trauma during implant placement is considered an important cause of implant failure.16,17 During a pilot hole osteotomy, most of the energy not used in the cutting process is transformed into heat. The amount of heat depends on the drill flute geometry,18,19 the sharpness of the cutting tool,20 the pressure applied,20 the duration of the cutting action,17,21 the cooling technique,22,23 the speed of the drill,24,25 and bone density.26 These factors are important because heat production leading to a temperature rise above 47°C for more than 1 minute negatively affects living bone27 and compromises its regeneration.28 It follows that placing a pilot hole for MSIs can generate enough heat to damage bone. In this event, the bone margins of the pilot hole undergo necrosis, followed first by resorption then osteogenesis. This results in the hole getting larger, which may lead to decreased stability of the MSI. Importantly, this is a delayed complication that occurs 1 to 3 weeks after placement. The probability increases in the posterior mandible where cortical bone is thicker and denser than most locations in the oral cavity.
My success rate with a non–drill-free MSI is 84.1%, whereas success is 97.5% with the drill-free Ortho Implant.
5) Why is immediate static loading with light forces important to ensure a high success rate?
Two terms have been used to describe loading of an implant earlier than traditional delayed loading (loading 6 weeks to 6 months after implant placement).29-33 Immediate loading has been defined as functional implant loading within 48 hours of surgical implant placement. Early loading has been defined as functional implant loading between 48 hours and 6 weeks after implant placement.34 Originally, based on BrÅnemark’s work,30-32 it was thought that all implants should undergo a 4- to 6-month healing period prior to functional loading. In the early 1990s, however, several groups found that the success of immediately loaded implants were comparable clinically and histologically to delayed-loaded implants as long as local micromotion was minimized.35,36
Dynamic loading is the term used to describe implant loading under intermittent loads of variable force levels, much as would be seen during mastication. These types of loads have been referred to as “jiggling forces” and are reported to increase implant failure.37 Static loading is the term used to describe constant loads of uniform force levels, similar to what is seen with orthodontic forces, usually loaded in one direction only, with a relatively uniform force over an extended period.
Duyck’s group38 demonstrated that statically loaded and unloaded controls showed a denser cortical lamellar bone at the neck and apex of the implants, whereas the dynamically loaded implants revealed bony craters and Howship’s lacunae around the implants necks, indicating a higher level of bony resorption. Based on some of the more relevant studies on dental implants and more recently on MSIs, it appears that implants can be loaded immediately as long as micromotion is minimized.39,40
My success rate with immediately loaded MSIs is 93.3%, whereas success is 64.3% with early loaded MSIs and 0% with delayed loaded MSIs.
6) What is important to know about placing an MSI near an extraction site?
Several authors have advocated a delay of 1 to 2 weeks before loading an MSI.2,41,42 This, however, is unnecessary and illogical, considering basic bone biology. Considering that the surgical placement of most MSIs does not involve more than a biopsy punch (as opposed to a true incision and flap, whether with or without a pilot hole), little to no postoperative discomfort or swelling occurs. The most critical factor is local bone healing, not so much for trauma caused by MSI placement but for other procedures performed in a similar time frame. For example, tooth extraction leaves a bony void. The body immediately mounts a response to fill the void. The detailed biologic response following extraction begins with a blood clot that is subsequently reorganized into callus tissue in which an initial scaffold of woven bone is formed. This is followed by reinforcement by parallel-fibered and/or lamellar bone. After a lag of several weeks, Haversian remodeling begins on the avascular areas of the socket wall and the newly formed tissue in the extraction site.43 Osseous regeneration starts approximately 3 weeks after extraction and is completed after 4 to 6 months.44-47 By the end of the first week, osteoclasts appear at the alveolar ridge and start to smooth the alveolar margin via resorption.48 This regressive restructuring is most pronounced in the first 4 weeks after extraction and then gradually declines until the fifth month.49
Considering the foregoing, as well as the regional acceleratory phenomenon associated with bony trauma,50-52 it is likely that a regional osteopenia occurs at sites adjacent to recent extraction sites. This can be explained as follows: As the body begins to infuse the extraction site with nutrients and cells to remove necrotic tissue and begin to build osteoid that is subsequently mineralized, it borrows minerals from adjacent bone, leading to a regional osteopenia. This may weaken adjacent bone and predispose MSIs placed simultaneously with dental extractions to inadequate primary stability and premature failure. In this scenario, it is advisable to wait 8 to 10 weeks after extraction for MSI placement in the same arch as an extraction.
My success rate when placing and loading an MSI within 8 weeks of an extraction site is 66.7%, whereas success is 93% if there has been no extraction or the extraction was done more than 8 weeks prior to MSI placement.
Criteria for Success
When I determine that I can’t treat a case with traditional orthodontic mechanics alone, and therefore need MSIs to provide anchorage (this is only about 20% of my total active cases), I use the following criteria to ensure success:
- Primary stability must be present or I move the MSI to another location.
- If at all possible, place the MSI in the palate or anterior to the first premolar in the upper or lower arch.
- Use the drill-free Ortho Implant; the only location in which I consider a pilot notch (a pilot hole that is less than 1 mm in depth) is in the retromolar area where bone is extremely thick and dense.
- Always load the MSI immediately with a light (50 g to 75 g) static load for the first 6 to 8 weeks.
- Delay placement of an MSI for at least 8 weeks after an extraction in the same arch.
Jason B. Cope, DDS, PhD, has a private practice in University Park, Tex. He is an adjunct clinical assistant professor at Baylor College of Dentistry. He is the author of OrthoTADs: The Clinical Guide and Atlas. He is a Diplomate of the American Board of Orthodontics and a member of the Edward H. Angle Society of Orthodontists. For more information on 1-day, 2-day hands-on, and Copesthetic in-office courses, Cope can be contacted at
Parts of the text were excerpted with permission from Ortho TADs: The Clinical Guide and Atlas, www.orthotads.com.
References
- Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003;124:373-378.
- Cheng SJ, Tseng IY, Lee JJ, Kok S-H. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004;19:100-106.
- Dalstra M, Cattaneo PM, Melsen B. Load transfer of miniscrews for orthodontic anchorage. Orthodontics. 2004;1:53-62.
- Schnelle MA, Beck FM, Jaynes RM, Huja S. A radiographic evaluation of the availability of bone for placement of miniscrews. Angle Orthod. 2004;74:830-835.
- Costa A, Pasta G, Bergamaschi G. Intraoral hard and soft tissue depths for temporary anchorage devices. Semin Orthod. 2005;11:10-15.
- Poggio PM, Incorvati C, Velo S, Carano A. Safe zones: a guide for miniscrew positioning in the maxillary and mandibular arch. Angle Orthod. 2006;76:191-197.
- Kyung HM, Bae SM, Park HS, et al. Microimplant anchorage (MIA) in orthodontics: various application sites and their considerations. In: McNamara JA Jr, ed. Implants, Microimplants, Onplants, and Transplants: New Answers to Old Questions in Orthodontics. Ann Arbor: Center for Human Growth and Development, University of Michigan, 2005:69-88.
- Sowden D, Schmitz JP. AO Self-drilling and self-tapping screws in tat calvarial bone: An ultrastructural study of the implant interface. J Oral Maxillofac Surg. 2002;60:294-299.
- Ansell R, Scales J. A study of some factors which affect the strength of screws and their insertion and holding power in bone. J Biomech. 1968;1:279-302.
- Bechtol C. Internal fixation with plates and screws. In: Bechtol C, Ferguson A, Laing P, eds. Metals and Engineering in Bone and Joint Surgery. Baltimore, Md: William & Wilkins, 1959:152-171.
- Kim J, Ahn S, Chang Y. Histomorphometric and mechanical analyses of the drill-free screw as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2005;128:190-194.
- Heidemann W, Gerlach KL, Grobel KH, Kollner HG. Drill free screws: a new form of osteosysthesis. J Craniomaxillofac Surg. 1998;26:163-168.
- Heidemann W, Terheyden H, Gerlach KL. Analysis of the osseous/metal interface of drill free screws and self-tapping screws. J Craniomaxillofac Surg. 2001;29:69-74.
- Nunamaker DM, Perren SM. Force measurements in screw fixation. J Biomech. 1976;9:669-675.
- Phillips JH, Rahn BA. Comparison of compression and torque measurements of self-tapping and pre-tapped screws. Plast Reconstr Surg. 1989;83:447-456.
- Lundskog J. Heat and bone tissue: an experimental investigation of the thermal properties of bone tissue and threshold level for thermal injury. Scand J Plast Renconstr Surg. 1972;6:5-75.
- Albrektsson T, Eriksson R. Thermally induced bone necrosis in rabbits: relation to implant failure in humans. Clin Orthop. 1985;195:311-312.
- Jacobs C, Pope M, Berry J, Hoagland F. A study of the bone machining process: orthogonal cutting. J Biomech. 1974;7:131-136.
- Wiggins K, Malkin S. Drilling of bone. J Biomech. 1976;9:553-559.
- Adell R, Lekholm U, BranËmark PI. Surgical procedures. In: BranËmark PI, Zarb GA, Albreksson T, eds. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago, Ill: Quintessence, 1985:211-232.
- Ågren E, Arwill T. High speed or conventional dental equipment for the removal of bone in oral surgery, III: a histologic and microradiographic study on bone repair in the rabbit. Acta Odontol Scand. 1968;26:223-246.
- Eriksson R, Albreksson T. Heat caused by drilling cortical bone: temperature measured in vivo in patients and animals. Acta Odontol Scand. 1984;55:629-631.
- Lavelle C, Wedgewood D. Effect of internal irrigation on frictional heat generated from bone drilling. J Oral Surg. 1980;38:499-503.
- Bolla E, Muratore F, Carano A, Bowman S. Evaluation of maxillary molar distalization with the distal jet: a comparison with other contemporary methods. Angle Orthod. 2002;72:481-494.
- Costich E, Youngblood P, Walden J. A study of the effect of high speed rotary instruments on bone repair in dogs. Oral Surg Oral Med Oral Pathol. 1964;17:563-571.
- Yacker M, Klein M. The effect of irrigation on osteotomy depth and bur diameter. Int J Oral Maxillofac Implants. 1996;11:634-638.
- Eriksson R, Albreksson T. Temperature threshold level for heat-induced bone tissue injury: a vital-microscope study in the rabbit. J Prosthet Dent. 1983;50:101-107.
- Thompson H. Effect of drilling into bone. J Oral Surg. 1958;16:22-30.
- Barewal RM, Oates TW, Meredith N, Cochran DL. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18:641-651.
- BrÅnemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses, I: experimental studies. Scand J Plast Renconstr Surg. 1969;3:81-100.
- BrÅnemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399-410.
- Adell R, Lekholm U, Rockler B, BrÅnemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387-416.
- Roberts WE, Smith RK, Zilberman Y, Moszary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod Dentofacial Orthop. 1984;86:95-111.
- Ganeles J, Wismeijer D. Early and immediately restored and loaded dental implants for single-tooth and partial-arch applications. Int J Oral Maxillofac Implants. 2004;19:92-102.
- Lum LB, Beirne R, Curtis DA. Histologic evaluation of hydroxylapatite-coated versus uncoated titanium blade implants in delayed and immediately loaded applications. Int J Oral Maxillofac Implants. 1991;6:456-462.
- Tarnow DP, Emitaz S, Classi A. Immediate loading of threaded implants at stage 1 surgery in edentulous arches: ten consecutive case reports with 1- to 5-year data. Int J Oral Maxillofac Implants. 1997;12:319-324.
- Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. J Biomed Mater Res. 1998;43:192-203.
- Duyck J, Ronold HJ, Van Oosterwyck H, Naert I, Vander Sloten J, Ellingsen JE. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: an animal experimental study. Clin Oral Implants Res. 2001;12:207-218.
- Costa A, Raffaini M, Melsen B. Miniscrews as orthodontic anchorage: a preliminary report. Int J Adult Orthodon Orthognath Surg. 1998;13:201-209.
- Freudenthaler JW, Haas R, Bantleon HP. Bicortical titanium screws for critical orthodontic anchorage in the mandible: a preliminary report on clinical applications. Clin Oral Implants Res. 2001;12:358-363.
- Liou EJ, Pai BCJ, Lin JC. Do miniscrews remain stationary under orthodontic forces? Am J Orthod Dentofacial Orthop. 2004;126:42-47.
- Park HS, Kwon OW, Sung JH. Uprighting second molars with micro-implant anchorage. J Clin Orthod. 2004;38:100-103.
- Schenk RK, Hunkizer EB. Histologic and ultrastructural features of fracture healing. In: Brighton CT, Friedlaender G, Lane JM, eds. Bone Formation and Repair. Rosemont, Ill: American Academy of Orthopedic Surgeons, 1994:117-146.
- Amler M. The time sequence of tissue regeneration in human extraction wounds. J Oral Surg. 1969;27:309-318.
- Amler M. The age factor in human extraction wound healing. J Oral Surg. 1977;35:193-197.
- Amler M, Johnson P, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:32-44.
- Simpson H. The healing of extraction wounds. Br Dent J. 1969;126:550-557.
- SchrÖder HE. Pathobiologie Oraler Srukturen. Basel, Switzerland: Karger, 1983.
- Lam R. Contour changes of the alveolar processes following extractions. J Prosthet Dent. 1960;10:25-32.
- Frost HM. The biology of fracture healing: an overview for clinicians, II. Clin Orthop. 1989:294-309.
- Frost HM. The biology of fracture healing: an overview for clinicians, I. Clin Orthop. 1989;283-293.
- Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983;31:3-9.









