The commercial campaign launch aims to educate orthodontic patients and parents on the deficiencies of traditional dental wax from a product safety and compliance perspective.
OrVance has released a 30-second oral health commercial campaign as part of their efforts to educate orthodontic patients and parents on the deficiencies of traditional dental wax from a product safety and compliance perspective.
Dubbed “Ax the Wax,” this campaign follows research by Michigan State University advertising classes highlighting the importance of educating parents on the deficiencies of dental wax and a May letter signed by a coalition of over 100 dentists and orthodontists to the FDA, and spearheaded by OrVance, requesting action against companies that continue to sell misbranded dental wax to orthodontic practices.
According to OrVance chief executive officer Ron Schutt, “Although this ad campaign is directed to orthodontic patients and their parents, it is also intended to call on the identified companies that continue to sell this product to do the right thing. In a Coalition Letter to the FDA back in May, we have identified 25 companies that continue to sell misbranded dental wax. This letter has been uncontested and not one of these companies has stepped forward to defend why the dental wax they sell should be exempt from current product safety and regulatory standards. We need more accountability against the identified importers and distributors that continue to push this substandard product throughout our industry.”
Orthodontist and OrVance co-founder Eric Hannapel, DDS, MS, PC, added, “Dental wax is still the most commonly dispensed product in our profession where most of our patients are children, so it’s unfortunate that we need a consumer advertising campaign to bring this issue to light. We believe it’s time these companies either address their noncompliance or explain to practices and the public why they believe this product doesn’t need to meet such basic product safety standards such as hygienic packaging, tamper evidence, disclosure of ingredients, product traceability and FDA required labeling. On a positive note, I want to applaud industry leaders such as GC Orthodontics that are partnering with us to fund the replacement of noncompliant dental wax for the health and safety of our patients.”
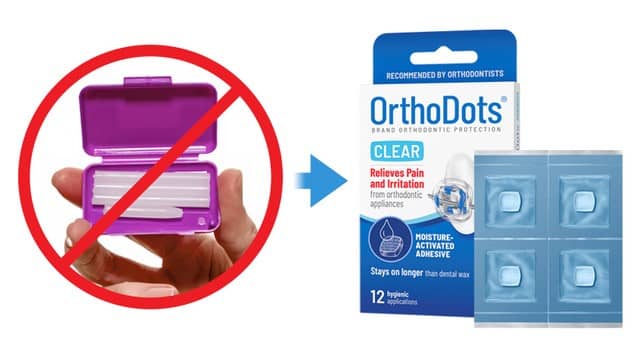
As part of the campaign, OrVance is encouraging patients and parents to ask their orthodontist about OrthoDots CLEAR, the company’s dental wax alternative. The company is offering orthodontic practices free samples of OrthoDots CLEAR through its website.