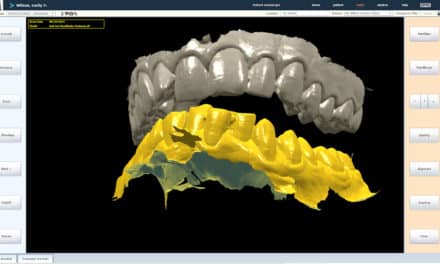
Summary: DentalMonitoring has launched a software update introducing new FDA-approved features, including SmartSTL, which enables remote monitoring and streamlined orthodontic workflows without the need for in-office scans.
Key Takeaways:
- FDA-approved software supports orthodontic treatment tracking with AI-powered remote monitoring.
- The SmartSTL feature simplifies aligner corrections and retainer creation by providing production-ready STL files remotely.
DentalMonitoring has introduced a significant software update with new features and indications following its De Novo approval from the FDA in May 2024.
DentalMonitoring’s remote monitoring solution uses advanced image processing algorithms to analyze intraoral scans taken by patients using the DentalMonitoring app and the ScanBoxpro phone holder. The AI-powered software provides clinical indications to remotely detect, track, and monitor orthodontic treatment progress, supporting real-time decision-making and personalized treatment.
READ MORE: SmartSTL Tech From DentalMonitoring Signals a New Era
Clinical Validation and FDA-Approved Indications
A clinical study program involving over 2,650 patients and more than 13,000 clinical results validated the safety and effectiveness of DentalMonitoring for tracking intraoral conditions and treatment progress. FDA-approved indications include:
- Archwire passivity
- Bracket debonding
- Tie loss
- Aligner seats/unseats
- Retainer seats/unseats
- Midline deviation
- Overbite, open bite, and overjet
This approval enhances the capability of orthodontic professionals to track treatment progress between in-office visits.
Introduction of SmartSTL Feature
The update also brings the SmartSTL feature to the United States, enabling orthodontists to request updated STL files through the DentalMonitoring dashboard. This eliminates the need for in-office intraoral scans for midcourse aligner corrections or retainer creation, streamlining workflows.
“We are proud to be the new standard of care in orthodontics. This is a recognition of our 10 years of research and development in Deep Learning Artificial Intelligence,” said Philippe Salah, CEO and co-founder of DentalMonitoring. “The De Novo FDA approval of this improved version of DentalMonitoring software is a key milestone for DentalMonitoring and makes DentalMonitoring the first and only Software as a Medical Device to monitor remotely orthodontic treatments.”
Photo via DentalMonitoring