This FDA market clearance includes 3Shape’s digital indirect bonding placement and transfer media application (IDB), as well as appliance design and production workflows.
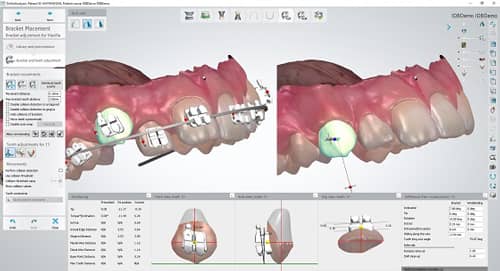
3Shape Ortho System allows orthodontic users to overlay DICOM, cephalometric, and 2D pictures along with intraoral scans for orthodontic case analysis and planning, treatment simulations, and the design and production of FDA-cleared orthodontic appliances.
When using 3Shape Ortho System, users can bring a patient’s malocclusion up onscreen and, with a few clicks, the software will suggest an ideal setup. This digital setup can be used in the planning of an indirect bonding bracket treatment as well as in creating the transfer media.
Over 275 bracket libraries are integrated with the 3Shape Ortho System software.
Like all 3Shape solutions, 3Shape Ortho System is open.