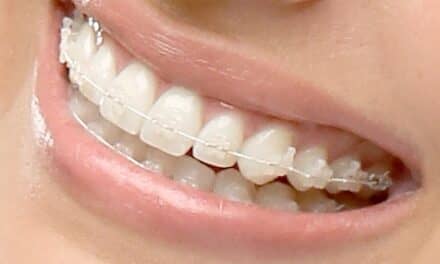
Insignia allows orthodontists to choose traditional twins, self-ligating brackets, or aligners that are customized to the individual patient. Compatible appliances include Insignia™ Clearguide™ Express aligners, Damon® Q™, Damon Clear, Titanium Orthos™, and Inspire ICE™.
Insignia uses a high-resolution scan of a PVS impression to create a 3D virtual model of the patient’s dental anatomy. According to the company, the system uses more than 40,000 data points per tooth, as well as unique algorithms in the software to define, coordinate, and design the patient’s arches into the best possible occlusion. The orthodontist then uses this initial occlusion to apply a more finite level of design, taking into consideration facial features and treatment preferences to create the ideal treatment plan.
Fixed appliances are delivered in Precision Placement Guides or jigs.
Ormco also provides an Insignia consumer education campaign, which includes a number of search engine marketing and PR programs to drive prospective patients to www.insigniasmile.com, where they will be able to locate an Insignia doctor. The company also offers turnkey in-office consultation materials ranging from lobby videos to TC presentations.
For more information about this and other orthodontic companies, visit our [removed]Buyer’s Guide[/removed].