by Jason B. Cope, DDS, PhD; John W. Graham, DDS, MD; Sebastian Baumgaertel, DMD, MSD, FRCD(C); S. Jay Bowman, DMD, MSD; Jack Fisher, DMD; James J. Hilgers, DDS, MS; Sarandeep S. Huja, DDS, PhD; David E. Paquette, DDS, MS, MSD; Mohammad R. Razavi, DDS, MSD, FRCD(C); Nicole Scheffler, DDS, MS; and Paul M. Thomas, DMD, MS, FDSRCS (Edin)
What orthodontists do and don’t need to do when placing MSIs
 |
Abstract
With the advent of new technologies in orthodontics come new protocols for implementing those technologies. Miniscrew implants (MSIs), the most popular category of temporary anchorage devices (TADs), are no different. As more orthodontists engage this innovative technology, new questions arise. Is this a surgical procedure? Can I perform MSI placement in my office? Do I need any special equipment? Are sterilization procedures different from traditional orthodontic procedures?
Unfortunately, these questions have been answered most frequently by opinion rather than fact. Therefore, the purpose of this article is to determine 1) if the MSI placement procedure is considered surgical or nonsurgical, 2) what federal agency regulates sterilization guidelines for these procedures, 3) who enforces infractions of these guidelines, and 4) what are appropriate sterilization guidelines based on scientific evidence.
Briefly, since the placement of an MSI—if placed without an incision, a flap, or a pilot hole—is nonsurgical, the Centers for Disease Control and Prevention (CDC) recommends sterilization guidelines but cannot enforce them, and infractions of sterilization guidelines are enforced by the individual state dental boards. The recommendations for MSI placement include hand-washing with an antimicrobial hand soap prior to donning clean medical exam gloves and using sterile instruments kept in a self-sealing sterilization container prior to placing a sterile MSI delivered from the manufacturer in a single-dose unit sterile package.
 |
| Jason B. Cope, DDS, PhD |
Introduction
Recently, Scholz and Cook1 authored “Sterilization Requirements for the Placement of Temporary Anchorage Devices,” in which the second author reported the sterilization requirements for TADs. Cook stated that “according to the CDC, the placement of a miniscrew in an orthodontic office is a surgical procedure.” Unfortunately, this statement has no veracity. The most recent CDC Guidelines were published in 2003, before TADs were in the orthodontic mainstream, and in fact, the CDC has no policy on TADs. To verify, the first author herein (JBC) called the CDC hotline on April 27, 2009, to inquire if the CDC had created a policy for orthodontic TADs. The CDC representative reviewed the policy manual and stated that the CDC did not have a specific policy for orthodontic TADs. The first author (JBC) again called the CDC on July 27, 2009, and spoke to a senior official in the Surveillance, Investigation, and Research Branch of the Division of Oral Health, who also stated that the CDC did not have a specific policy for TADs or MSIs.
 |
 |
| Figure 1: Self-sealing sterilization pouches with integrated chemical indicators. Left is before sterilization. Note the light blue arrow (circled). Right is after sterilization. Note the dark purple arrow (circled). | |
Further, Cook detailed the guidelines for providing sterile instruments for TAD procedures. These guidelines are also incorrect. It appears her assumption is that all miniscrew implants are delivered from the manufacturer nonsterile, and hence must undergo a special, more stringent protocol for sterilization than miniscrew implants delivered from the manufacturer sterile. While it is true that some manufacturers provide nonsterile implants, most miniscrew implants manufactured in the United States are delivered from the manufacturer to the clinician in a sterile condition, and are therefore held to different sterilization standards. Finally, the statement that “orthodontists have been firm in stating their sterilization needs: TAD placement is not a surgical procedure, so sterile instruments are unnecessary” is only partially correct. Yes, we agree that TAD placement is not a surgical procedure; however, we disagree that sterile instruments are unnecessary, as explained below. Moreover, a review of the article reveals no single reference from which data was collected to base the statement about orthodontists’ beliefs.
Considering this, and many other incorrect assumptions asserted in the article, we decided to review the pertinent OSHA, FDA, CDC, ADA, and AAO policies and present the facts as well as prudent sterilization guidelines for MSI placement for the protection of our patients.
 |
| John W. Graham, DDS, MD |
OSHA
OSHA, which is an agency under the direction of the US Department of Labor, specifically “governs the safety of workplace employees and not sterilization procedures for dental health care personnel or patients. Under the Occupational Safety and Health Act of 1970, OSHA’s role is to ensure safe and healthful working conditions for working men and women by authorizing enforcement of the standards developed under the Act; by assisting and encouraging the States in their efforts to ensure safe and healthful working conditions; and by providing for research, information, education, and training in the field of occupational safety and health.”2
According to OSHA,3 there are currently no specific standards for dentistry. However, exposure to numerous biological, chemical, environmental, physical, and psychological workplace hazards that may apply to dentistry are addressed in specific standards for the general industry. Section 5(a)(1) of the OSH Act, often referred to as the General Duty Clause, requires employers to “furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees.” Section 5(a)(2) requires employers to “comply with occupational safety and health standards promulgated under this Act.”
 |
| S. Jay Bowman, DMD, MSD |
FDA
The FDA, which is one of the federal agencies within the Department of Health and Human Services, “is responsible for protecting the public health by ensuring the safety, efficacy, and security of medical devices; human and veterinary drugs; biological products; our nation’s food supply; cosmetics; and products that emit radiation.”4 The FDA also ensures that these products are honestly, accurately, and informatively represented to the public. Specific responsibilities with regard to medical devices include premarket approval of new devices, manufacturing and performance standards, and tracking reports of device malfunctioning and serious adverse reactions.5 Therefore, the FDA does not regulate sterilization procedures for dental health care personnel or patients.
CDC
The CDC, which is another agency within the Department of Health and Human Services, is the nation’s disease prevention agency. It “develops a broad range of guidelines intended to improve the effect and effectiveness of public health interventions and to inform clinicians, public health practitioners, and the public. The CDC strives to ensure that guidelines are clear, practical, and evidence-based.” The CDC, unlike OSHA (which is a regulatory agency), cannot mandate certain practices; it can only recommend. Nevertheless, many dental licensing boards adopted the CDC’s recommendations, or variations of them, as the infection control standard for dental practice in their states.6
For example, the Texas State Board of Dental Examiners Rules and Regulations, Chapter 108, Subchapter B states that its policy is that of the ADA and CDC, namely: "(a) Sterilization is required for all surgical and other instruments that may be used intraorally or extraorally, where these instruments may be used invasively or in contact with or penetration of soft tissue, bone, or other hard tissue. Other nonsurgical instruments, such as plastic instruments, that may come into contact with tissue must be disinfected with an American Dental Association-registered solution that is tuberculocidal. (b) All instruments subject to sterilization must undergo at least one of the following procedures: (1) steam autoclave, (2) chemical vapor, (3) dry-heat oven, (4) ethylene oxide, or (5) chemical sterilant.”7
 |
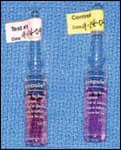 |
 |
| Figure 2: Incubator and self-contained sterilization vials containing biologic indicators. Left is a dry block incubator. Center is before sterilization. Note that both vials contain purple broth and only the test vial is run through the sterilizer. Right is after sterilization. Note that the test vial is still purple, indicating lack of bacterial spore growth, and the control vial is now yellow, indicating bacterial spore growth. | ||
The most recent guidelines by the CDC were published in 2003.8 This report consolidates recommendations for preventing and controlling infectious diseases and managing personnel health and safety concerns related to infection control in dental settings. The report also 1) updates and revises previous CDC recommendations regarding infection control in dental settings, 2) incorporates relevant infection-control measures from other CDC guidelines, and 3) discusses concerns not addressed in previous recommendations for dentistry.
 |
| Jack Fisher, DMD |
ADA
The ADA Statement on Infection Control in Dentistry “urges all practicing dentists, dental auxiliaries, and dental laboratories to employ appropriate infection control procedures as described in the 2003 CDC Guidelines, and to keep up to date as scientific information leads to improvements in infection control, risk assessment, and disease management in oral health care.” Therefore, the ADA recommends using the CDC guidelines, but defers to the individual state dental boards for definitive regulatory information and enforcement.9
AAO
Although the AAO has no official statement on the placement of MSIs as being surgical or nonsurgical, evidence that the American Association of Orthodontists Insurance Company (AAOIC) does not consider the placement of an MSI a surgical procedure lies in the fact that the AAOIC Liability Insurance Policy10 excludes any and all surgical procedures, yet it includes coverage for the placement of a MSI. Specifically, Section II, “Exclusions” reads, “In addition to other limitations of this policy, this insurance does not apply: I. To any injury resulting from the performance of any surgical procedure or surgical extraction or the performance of a non-surgical extraction. This exclusion does not apply to: 5. The placement of micro implants that do not involve the reflection of a surgical flap.” It follows that the AAOIC also does not consider MSI placement a surgical procedure as long as a surgical flap is not raised.
 |
| Sebastian Baumgaertel, DMD, MSD, FRCD(C) |
CDC Guidelines
Considering that OSHA and the FDA do not mandate sterilization procedures, the CDC publishes guidelines but doesn’t have the jurisdiction to enforce them, and the ADA and AAO recommend following the CDC guidelines, it appears that a detailed review of the CDC guidelines is in order. Moreover, the individual state dental boards, which do have enforcement jurisdiction over OSHA violations and CDC guidelines, also recommend following the CDC guidelines.7
Upon reviewing the 66 pages of the CDC’s 2003 Guidelines, it appears that the primary criterion upon which to base sterilization guidelines for MSIs should be the CDC’s definition of oral surgical procedures. Based on whether the procedure is surgical or nonsurgical, then orthodontists should follow the specific CDC sterilization guidelines for that particular defined procedure. In addition, separate sterilization procedures are required depending on whether the MSI comes from the manufacturer in a sterile or nonsterile condition.
Oral Surgical Procedures, Page 32
Oral surgical procedures involve the incision, excision, or reflection of tissue that exposes the normally sterile areas of the oral cavity. Examples include biopsy, periodontal surgery, apical surgery, implant surgery, and surgical extractions of teeth (e.g., removal of erupted or nonerupted tooth requiring elevation of mucoperiosetal flap, removal of bone or section of tooth, and suturing if needed).
The term “TAD” covers a broad category of devices, including MSIs, miniplate implants (MPIs), and palatal implants (PIs), to name a few.11 A miniscrew implant is a subcategory of TAD that is placed and used completely differently from miniplate implants and palatal implants. For example, the authors recommend a minimally invasive miniscrew implant placement protocol including topical anesthesia, no incision or flap, and no pilot hole prior to placing a drill-free, self-tapping MSI. Miniplate implants require local anesthetic, an incision and flap; palatal implants require local anesthetic, an incision and flap, and a full-depth pilot hole. Miniplate implant and palatal implant placement are surgical procedures; MSI placement is not a surgical procedure. Therefore, the remainder of this article will focus entirely on the use of miniscrew implants.
 |
| Sarandeep S. Huja, DDS, PhD |
It is clear from the CDC’s definition of Oral Surgical Procedures that it intends the definition to include surgical procedures that incise, excise, or reflect soft or hard tissue. Placement of an MSI does not require this. Further, the CDC gives examples of surgical procedures that include biopsy, in which an incision is made, tissue is removed for biopsy, and the resulting wound is sutured closed. Another example the CDC cites is implant surgery, for which standard procedures include an incision, reflection of a mucoperiosteal flap, drilling of a full-depth pilot hole with sterile saline irrigation, placement of the dental implant, and finally suturing of the soft tissues around the implant. The final example given is that of a surgical extraction, for which the CDC parenthetically requires elevation of a mucoperiosetal flap, removal of bone or section of tooth, and suturing if needed. It follows that the placement of an MSI would not fall under the category of oral surgical procedures. Moreover, based on the lack of inclusion of a nonsurgical extraction in the list of surgical examples, the CDC apparently considers the nonsurgical extraction of a tooth (wherein there is no incision or elevation of a mucoperiosetal flap, removal of bone or sectioning of a tooth, or suturing) nonsurgical. Clearly, a nonsurgical extraction is more invasive than the placement of an MSI. Taken together, this information indicates that the placement of an MSI is nonsurgical. It should also be pointed out that, to the collective best knowledge of the authors, there has been no published report of an increased infection rate associated with MSI placement.
Based on the argument that the placement of an MSI is nonsurgical, the following CDC guidelines are appropriate for MSIs purchased from the manufacturer in a single-dose unit sterile package: hand hygiene, personal protective equipment, and sterilization and disinfection of patient care items:
III. Hand Hygiene, Page 41
- Perform hand hygiene with either a nonantimicrobial or antimicrobial soap and water when hands are visibly dirty or contaminated with blood or other potentially infectious material. If hands are not visibly soiled, an alcohol-based hand rub can also be used. Follow the manufacturer’s instructions.
- Indications for hand hygiene include a. when hands are visibly soiled; b. after barehanded touching of inanimate objects likely to be contaminated by blood, saliva, or respiratory secretions; c. before and after treating each patient; d. before donning glove; and e. immediately after removing gloves.
 |
| David E. Paquette, DDS, MS, MSD |
Items 1 and 2 are the same precautions for any dental or orthodontic procedure and should be followed as recommended. This is no different than what orthodontists should already be doing in their offices on every procedure.
IV. PPE, Page 41
A. Masks, Protective Eyewear, and Face Shields
- Wear a surgical mask and eye protection with solid side shields or a face shield to protect mucous membranes of the eyes, nose, and mouth during procedures likely to generate splashing or spattering of blood or other body fluids.
B. Protective Clothing
- Wear protective clothing (e.g., reusable or disposable gown, laboratory coat, or uniform) that covers personal clothing and skin (e.g., forearms) likely to be soiled with blood, saliva, or Other Potentially Infectious Materials (OPIM).
C. Gloves
- Wear medical gloves when a potential exists for contacting blood, saliva, OPIM, or mucous membranes.
“PPE” refers to personal protective equipment, for which there is no added requirement over what is normally done in an orthodontic office. Masks, protective eyewear, face shields, and protective clothing are only required during procedures likely to generate splashing or spattering of blood or other body fluids. Likewise, medical gloves should be worn when a potential exists for contacting blood, saliva, OPIM, or mucous membranes. This is no different from what orthodontists should already be doing for every procedure in their offices.
Special consideration should also be given here to nonsterile, but clean, gloves. Three randomized, prospective studies12-14 were performed to compare postoperative complications and infections when surgeons were wearing sterile versus nonsterile, but clean, gloves during nonsurgical extractions. In all three studies, collectively totaling 521 patients who had teeth extracted and had no postoperative antibiotics prescribed, there were no statistical differences in infection rates. The authors concluded that nonsurgical dental extractions can be safely performed with the surgeon wearing clean, nonsterile gloves. It follows that the same would be true for MSI placement.
 |
| Mohammad R. Razavi, DDS, MSD, FRCD(C) |
VI. Sterilization and Disinfection of Patient-Care Items, Page 42
A. General Recommendations
- Use only FDA-cleared medical devices for sterilization and follow the manufacturer’s instructions for correct use.
- Clean and heat-sterilize critical dental instruments before each use.
D. Preparation and Packaging
- Use an internal chemical indicator in each package. If the internal indicator cannot be seen from outside the package, also use an external indicator.
- Use a container system or wrapping compatible with the type of sterilization process used and that has received FDA clearance.
F. Sterilization Monitoring
- Use mechanical, chemical, and biological monitors according to the manufacturer’s instructions to ensure the effectiveness of the sterilization process.
- Monitor each load with mechanical (e.g., time, temperature, and pressure) and chemical indicators.
- Place a chemical indicator on the inside of each package. If the internal indicator is not visible from the outside, also place an exterior chemical indicator on the package.
- Monitor sterilizers at least weekly by using a biological indicator with a matching control (i.e., biological indicator and control from same lot number).
G. Storage Area for Sterilized Items and Clean Dental Supplies
- Implement practices on the basis of date- or event-related shelf-life for storage of wrapped, sterilized instruments and devices.
- Examine wrapped packages of sterilized instruments before opening them to ensure the barrier wrap has not been compromised during storage.
- Store sterile items and dental supplies in covered or closed cabinets, if possible.
The only difference between sterilizing normal orthodontic instruments and TAD-related instruments is that we recommend that TAD-related instruments be sterilized separately in either a cassette sealed in a self-sealing sterilization pouch, or individually in a self-sealing sterilization pouch. The rationale for this is that, similar to a nonsurgical extraction, the procedure is slightly more invasive, and, as such, the preferred method is to sterilize those instruments in a sealed container.
In addition to the above guidelines, the following CDC guidelines apply if an MSI is purchased from the manufacturer in a nonsterile condition.
VI. Sterilization and Disinfection of Patient-Care Items
E. Sterilization of Unwrapped Instruments
- Do not sterilize implantable devices unwrapped
F. Sterilization Monitoring
- Use a biological indicator for every sterilizer load that contains an implantable device. Verify results before using the implantable device, whenever possible.
 |
| Nicole Scheffler, DDS, MS |
If the clinician is responsible for sterilizing the MSI (instead of purchasing an MSI in a single-dose unit sterile package), then a biological indicator should be used for every sterilizer load that contains an implantable device, and the sterilization results should be verified before using the MSI.
At this point, the difference between a chemical and a biological indicator should be clarified.
Chemical indicators change color or physical form when exposed to elevated temperatures.15 The color or form change is immediately observable upon completion of the sterilization cycle. There are two types of chemical indicators: surface indicators and integrated indicators. A surface indicator changes color or form when exposed to a specific elevated temperature. An integrated indicator, however, changes color or form more slowly, and responds to a combination of time and temperature or to a process involving time, temperature, and the presence of steam. Integrated indicators commonly are used on the inside of every pack, pouch, or cassette to ensure that the instruments inside the packaging material have all been exposed to sterilizing conditions (Figure 1). We recommend integrated chemical indicators over surface indicators.
Biological indicators contain bacterial spores that change color if the sterilization process fails.15 There are two types of biological indicators: spore strips and self-contained vials. The spore strip is enclosed in a protective glassine envelope. After processing through the sterilizer, the strip is aseptically removed from the envelope and placed in a tube of appropriate Tryptic-soy broth medium that in turn is incubated for 2 to 7 days at 55°C/131°F (for G. stearothermophilus) or at 37°C/98.6°F (for B. subtilis). If live bacterial spores are still present, they will grow and produce cloudiness and/or change the color of the growth medium, indicating sterilization failure. A self-contained vial includes both a spore strip or disk and an ampule filled with growth medium, contained in a plastic vial with a vented cap to permit entrance of the sterilizing agent into the vial. After running through the sterilizer, users either squeeze the vial or push the cap down to break the internal ampule, which mixes the growth medium with the spores. The vial is then incubated at 55°C/131°F, and if live bacterial spores still are present, they will grow and change the color of the growth medium, indicating sterilization failure. Biological indicators can either be analyzed in the office with the purchase of an incubator and the appropriate self-contained biological indicators (Figure 2 above), or by using spore strips and a broth culture system. Alternatively, the office can subscribe to a sterilization monitoring service that is available commercially and at some dental schools.15 Regardless of where they are analyzed, the incubation process takes from 2 to 7 days after the completion of the sterilization process before the results are known and the MSI can be placed.
 |
| Paul M. Thomas, DMD, MS, FDSRCS (Edin) |
According to the CDC Guidelines, a biological indicator should be used for every sterilizer load that contains an implantable device and the sterilization results should be verified before using the MSI.
Recommended MSI Sterilization Guidelines
Based on the above, we recommend the following sterilization guidelines for implementing MSI placement in clinical practice without the use of an incision, flap, or pilot hole. It should be pointed out that these guidelines are formulated based on our collective critical analysis and interpretation of the current CDC Guidelines as well as currently available scientific literature, and may be modified accordingly as new CDC Guidelines and scientific advances become available.
As mentioned previously, the individual states and their respective dental boards have the final jurisdiction over any apparent infraction. A prudent clinician would seek legal counsel in his or her own state as to the legal ramifications of these or any other guidelines put forth for MSI sterilization. We suggest that orthodontists use the following:
- A sterile MSI: many manufacturers sell MSIs that come in a single-dose unit sterile package, which eliminates the clinician having to sterilize the MSI and being held to more stringent CDC guidelines. MSIs purchased nonsterile must undergo separate, more stringent sterilization guidelines as outlined above.
- A self-sealing sterilization pouch: most of these come with an integrated chemical indicator embedded in the pouch, and when sterilized the indicator arrow turns from blue to purple.
- Sterile instruments: sterilized in either a self-sealing sterilization pouch or an instrument cassette enclosed in a self-sealing sterilization pouch.
- Antimicrobial hand soap: for hand washing prior to donning clean medical exam gloves.
- Powder-free medical exam gloves: the literature indicates no difference in infection rates with sterile as compared to clean, nonsterile gloves for nonsurgical extractions, which are more invasive than MSI placement.
It should be pointed out that, because the placement of miniplate implants and palatal implants fall under the category of surgical procedures, orthodontists would be obligated to follow the CDC’s surgical guidelines for sterilization, which are not considered here. Also, irrigation is not required for the placement of MSIs, so the use of sterile saline irrigation is not considered herein.
Jason B. Cope, DDS, PhD, is in private practice in Dallas and is an adjunct associate professor in the St Louis University Department of Orthodontics. He can be reached at
John W. Graham, DDS, MD, is in private practice in Litchfield Park, Ariz, and is adjunct associate professor for clinical orthodontics in the University of the Pacific’s Arthur A. Dugoni School of Dentistry.
Sebastian Baumgaertel, DMD, MSD, FRCD(C), is an assistant clinical professor in the department of orthodontics at Case Western Reserve University.
S. Jay Bowman, DMD, MSD, is in private practice in Portage, Mich. He is an adjunct associate professor at Saint Louis University and an instructor at the University of Michigan.
Jack Fisher, DMD, is private practice in Memphis, Tenn. He is an adjunct associate professor in the department of orthodontics at the University of Louisville School of Dentistry and an adjunct associate professor in the department of orthodontics at the New York University College of Dentistry.
James J. Hilgers, DDS, MS, is in private practice in Mission Viejo, Calif, and is an associate professor of orthodontics at the Loma Linda University School of Dentistry.
Sarandeep S. Huja, DDS, PhD, is an associate professor in the division of orthodontics at Ohio State University’s College of Dentistry.
David E. Paquette, DDS, MS, MSD, is in private practice in Charlotte, NC.
Mohammad R. Razavi, DDS, MSD, FRCD(C), is in private practice in Ottawa, Canada, and is an assistant clinical professor in the department of orthodontics at Case Western Reserve University.
Nicole Scheffler, DDS, MS, is in private practice in Boone, NC, and is an adjunct assistant clinical professor in the department of orthodontics at the University of North Carolina at Chapel Hill.
Paul M. Thomas, DMD, MS, FDSRCS (Edin), is an adjunct professor in the Department of Orthodontics at the University of North Carolina at Chapel Hill.
References
- Scholz RP, Cook A. Sterilization requirements for the placement of temporary anchorage devices. Am J Orthod Dentofac Orthop. 2009;135:S20-S22.
- OSHA’s Role. From website of US Dept of Labor: Washington, DC. Available at www.osha.gov/oshinfo/mission.html Accessed June 1, 2009.
- OSHA’s Standards. From website of US Dept of Labor: Washington, DC. Available at www.osha.gov/SLTC/dentistry/standards.html. Accessed June 1, 2009.
- Centers and Offices. From website of US Dept of Health and Human Services: Silver Spring, MD. Available at www.fda.gov/AboutFDA/CentersOffices/default.htm. Accessed June 1, 2009.
- What FDA Regulates. From website of US Dept of Health and Human Services: Silver Spring, MD. Available at [removed]www.fda.gov/AboutFDA/WhatWeDo/WhatFDARegulates/default.htm[/removed]. Accessed June 1, 2009.
- Kohn WG, Harte JA, Malvitz DM, Collins AS, Cleveland JL, Eklund KJ. Guidelines or infection control in dental settings – 2003. J Am Dent Assoc. 2004;135:33-47.
- Texas State Board of Dental Examiners Rules and Regulations. From website of State of Texas: Austin, Tex. Available at www.tsbde.state.tx.us/documents/rules/SBDE-Rules-Mar2009.pdf. Accessed June 1, 2009.
- Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Guidelines or infection control in dental health-care settings – 2003. Morbidity and Mortality Weekly Report; 2003:1-68.
- ADA statement on infection control in dentistry. From website of American Dental Association: Chicago. Available at [removed]www.ada.org/prof/resources/positions/statements/infectionconrol.asp[/removed]. Accessed June 1, 2009.
- Orthodontist Professional Liability Insurance Policy. Montpelier, VT: American Association of Orthodontists Insurance Company; 2008:1-16.
- Cope JB. Temporary anchorage devices in orthodontics: A paradigm shift. Semin Orthod. 2005;11:3-9.
- Giglio, Roland, Laskin, Grenevicki. The use of sterile versus nonsterile gloves during out-patient exodontia. Quintessence International. 1993;24:543-545.
- Chiu WK, Cheung LK, Chan HC, Chow LK. A comparison of post-operative complications following wisdom tooth surgery performed with sterile or clean gloves. Int J Oral Maxillofac Surg. 2006;35:174-179.
- Adeyemo WL, Ogunlewe MO, Ladeinde AL, Bamgbose BO. Are sterile gloves necessry in nonsurgical dental extractions. J Oral Maxillofac Surg. 2005;63:936-940.
- Miller CH. Sterilization: Instrumental in patient safety. From website of Alfa Medical: Westbury, NY. Available at www.sterilizers.com/articles/Sterilization-process.asp. Accessed June 1, 2009.









